In
quality-critical industries like pharmaceuticals, cosmetics, and water
treatment, microbial testing must balance speed, sensitivity, and regulatory
compliance. Traditional culture-based methods, although proven, require several
days—or even weeks—to yield results.
Enter
Red One™ – the
latest generation of automated solid-phase cytometry – now available in India
through BiomatiQ, your trusted partner for advanced microbiological solutions.
What is
Red One™?
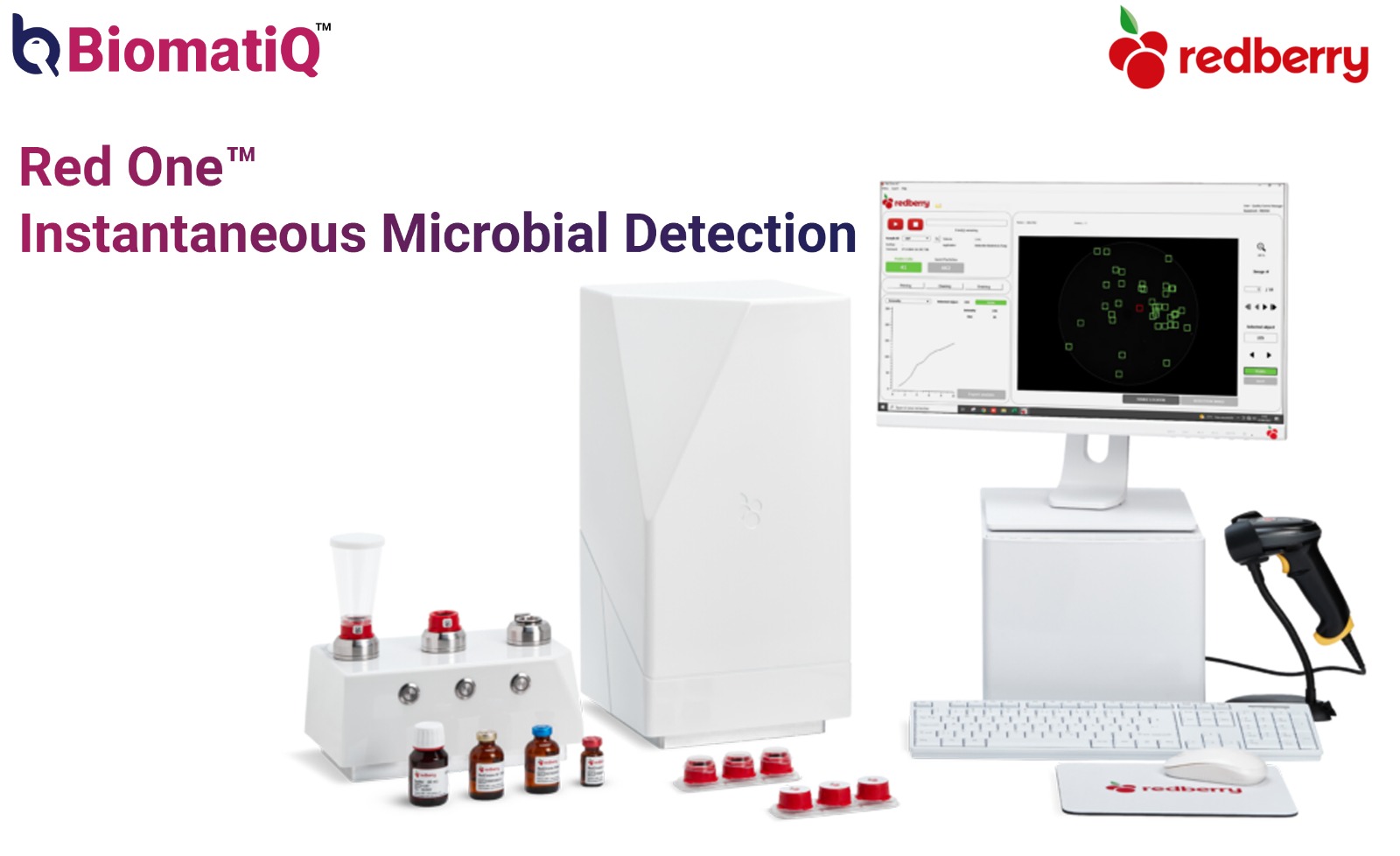
Red
One™ is a fully automated platform for detecting and enumerating viable
microorganisms in filterable liquid samples, including those rendered
filterable through pretreatment (e.g., rinsing or lysis). It identifies viable
single cells based on their esterase activity, using a fluorescent substrate.
With
applications across rapid screening, bioburden, and sterility testing, Red One™
delivers fast and highly reliable results—with no need for calibration.
Core
Advantages
- Sample Volume Range: 10 µL to 250 mL
- Single-Use Cap: Touch-free workflow reducing risk of contaminations
- Fully Automated: Filtration, staining and analysis – repeatable and robust procedure
- Calibration-Free Operation – reduces hands-on time and variability
- Compact Footprint: Integrates easily into a safety cabinet
Red
One™ Applications
✅ 1.
Rapid Screening in 10 Minutes
The Direct Viable Count application provides a total viable cell count in just 10
minutes. Simply drop the sample onto the disposable cap — and the system does
the rest.
• Detects as low as a few cells/sample
• Superior to ATP & Flow Cytometry (typically 1000 cells/mL LOD)
• Demonstrated consistency with traditional 5 Day counts on R2A plates for
water loops
• Other applications include in-process, environmental, or lot enumeration
✅ 2.
Bioburden Testing in 4 Hours
Designed for total aerobic flora including bacteria, fungi, and spores. After
the initial sample filtration onto the cap, a 4-hour activation step is
employed to restore the metabolic activity of the cells, making it compliant
with staining and detection.
• Quantitative bioburden result in just 4 hours
• Optional 24-hour enrichment step for 1 CFU sensitivity and post-analysis
identification
• Primary validation in accordance with European and US Pharmacopoeias will be
available starting September 2025
✅ 3.
Sterility Testing in Less Than 4 Days
A true game-changer, Red One™ performs qualitative sterility testing using standard
double-canister sample prep, with a time-to-result (TTR) of less than 4 days—a
major improvement over traditional 14-day methods.
• Compatible with compendial workflows
• Detects individual microbial cells, eliminating the need for high biomass
• Accelerates batch release and contamination investigations
• Full primary validation is already available, and customers receive
comprehensive support for rapid product-specific validation
Globally
Recognized Innovation
Red One™ has earned international recognition for its innovation and design:
Most Innovative Start-up in
Microbiology (2022)
– StartUs Insights Discovery Platform (out of 1,061 companies)
Best Product Design for
Healthcare (2020)
– Janus de la Santé, French Institute of Design
Ready
to Transform Your Microbial Testing?
Whether you're optimizing environmental monitoring, reducing time-to-release,
or increasing the reliability of sterility testing—Red One™ delivers.
Contact
BiomatiQ today to schedule
a demo, request a technical brochure, or speak to our application specialists.
contact@biomatiq.com
Toll Free No.: 1800 571
9696