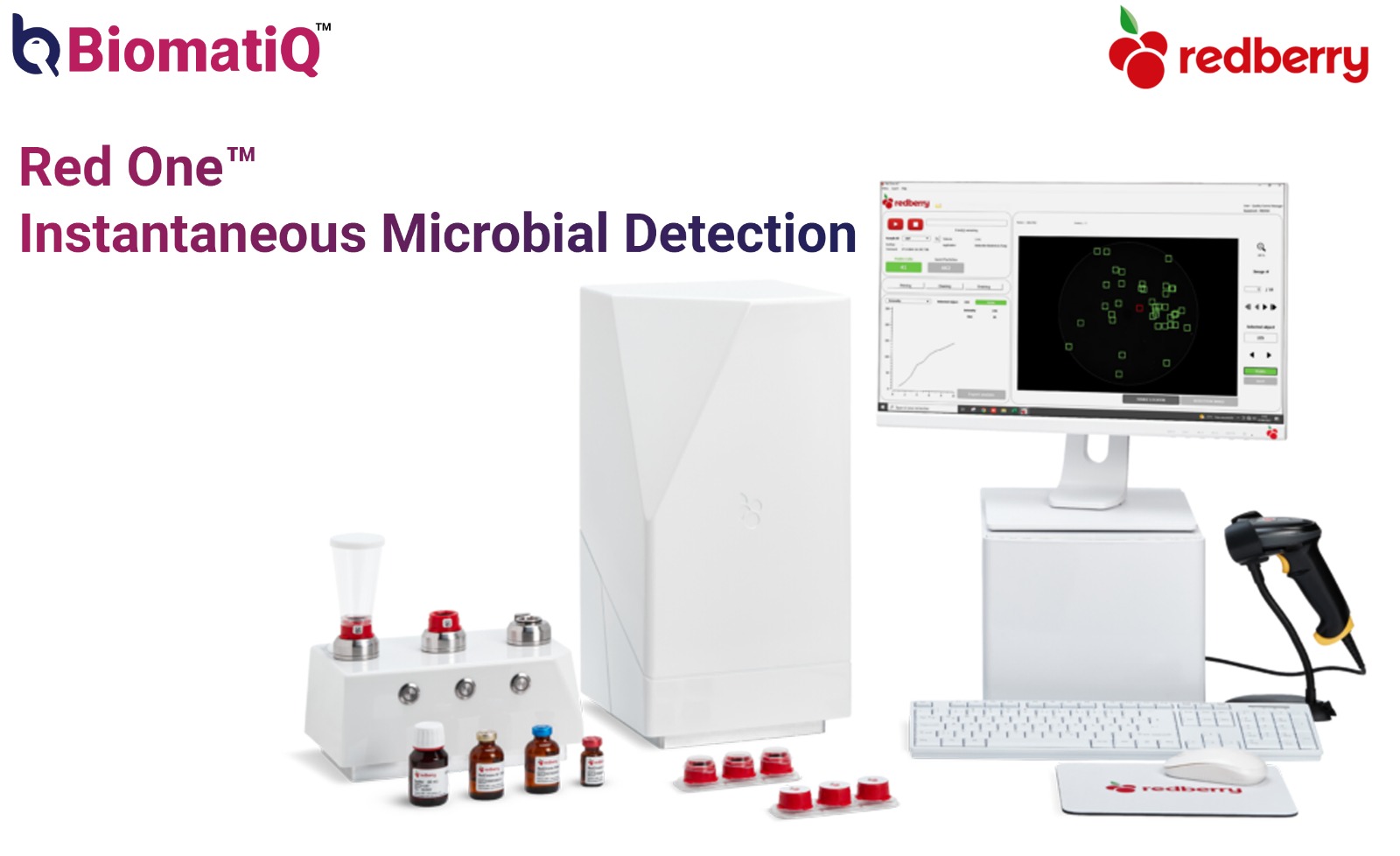
Environmental monitoring (EM) programs have relied on viable
air sampling as a core method for assessing microbiological cleanliness in
controlled environments. For professionals working in pharmaceutical,
biotechnology, and healthcare settings, the principles behind active air
sampling are well-established. However, what is changing rapidly are the
expectations placed on air samplers—not just as microbial collection devices,
but as data-compliant, digitally integrated tools.
In this context, it becomes critical to reassess what the
scientific community now needs from biological environmental air samplers.
Beyond Colony Counting: A New Role for Air Samplers
Historically, the role of active air samplers was limited to
drawing in ambient air, impacting it onto an agar plate, and enabling
colony-forming unit (CFU) enumeration. That function remains essential. But in
today’s regulatory and operational landscape, samplers are now part of a
broader digital and compliance ecosystem. They must support:
- 21
CFR Part 11 compliance for secure electronic data handling
- Integration
with LIMS/MODA systems
- Remote
operability, especially in automated cleanroom systems
Modern air samplers are no longer stand-alone instruments.
They are endpoints in a networked environment that demands robust data
governance, traceability, and system interoperability.
What the Modern Scientific User Expects
1. Data Integrity by Design
GMP environments now require instruments that offer:
- Unique
user IDs and access controls
- Time-stamped
audit trails
- Secure
electronic signatures
- Tamper-proof
storage (local or server-based)
These features must be native—not retrofitted—to ensure compliance from day one.
2. Interconnectivity with Quality Systems
Modern air samplers must communicate effectively with
enterprise digital quality systems. This includes:
- Bi-directional
communication with LIMS or middleware
- Automated
data exports in secure formats
- Configuration
sync (sampling programs, locations)
- Compatibility
with LDAP or Active Directory
In regulated facilities, the ability to pull data from and
push data to validated servers is essential for harmonizing environmental data
with broader quality systems.
3. Smart Alerts and Fail-Safes
Sampling errors—such as missing plates or improper head
assembly—can compromise entire monitoring cycles. Advanced samplers now
feature:
- Plate
presence detection
- Head
blockage and open-head alarms
- Pre-run
integrity checks
- Sampling
lockouts for calibration expiry
These features ensure sampling integrity and support
right-first-time execution.
Adaptability to Specific Monitoring Needs
The next generation of air samplers must be adaptable to a
wide range of operational setups. Scientific users increasingly seek:
- Variable
flow rate options (100, 180, 200 LPM)
- Compressed
gas sampling assemblies with regulators and diffusers
- Compatibility
with VHP sterilization, isolators, and RABS
- Sterile
disposable heads for critical environments
- Modular
configurations (Lite to enterprise-level) that scale with operational
growth
This adaptability ensures that one system can serve multiple
facilities, zones, and compliance levels.
The Overlooked Importance of Compressed Gas Monitoring
Compressed gases are often used in aseptic processes but are
frequently under-monitored. Microbial sampling from compressed gases should
follow best practices aligned with ISO 8573 and EU GMP, using:
- Pressure-regulated
diffusers
- HEPA
filtration where necessary
- Portable,
stainless-steel-compatible assemblies
- Easy
disinfection and sterilization
Microbial monitoring of gas lines must be integrated into
the overall EM program—not treated as an afterthought.
Air Samplers as Strategic Digital Assets
Looking ahead, environmental air samplers will be
increasingly viewed not just as instruments, but as digital quality assets
that:
- Enable
real-time traceability
- Reduce
human error and documentation gaps
- Deliver
actionable insights through trend data
- Support
integrated contamination control strategies
As regulatory scrutiny intensifies and data governance
becomes paramount, the choice of air sampler can significantly impact an
organization’s compliance posture, operational efficiency, and long-term risk
mitigation.
A Subtle Shift in the Market
Innovative air sampling platforms—such as the modular,
digitally enabled systems offered by BiomatiQ’s AirQ BEAS series—are
reflecting these trends. With models that range from basic monitoring to fully
21 CFR Part 11 and LDAP-integrated configurations, they illustrate how samplers
can evolve to meet the modern demands of scientific EM programs.
For professionals navigating the complex intersection of
microbiology, compliance, and digital quality, the future of air sampling is
here—and it's smarter, safer, and more connected than ever.